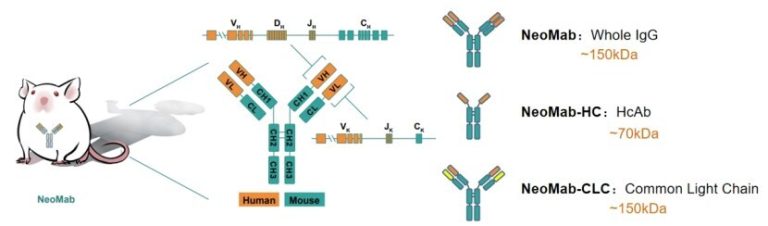
NeoMab Biotechnology (Suzhou) Co., Ltd. (commonly referred to as “NeoMab”) has launched its cutting-edge NeoMab-IgG Ease model, an advanced fully human antibody transgenic mouse model. This innovative platform signifies a major advancement in antibody drug development, enabling researchers and pharmaceutical companies to streamline their processes in discovering and developing fully human antibodies efficiently.
Simplifying Antibody Development
The NeoMab-IgG Ease model introduces a revolutionary method for antibody drug development by offering a distinctive “milestone-free, live mouse provision” framework. This flexible purchasing structure allows commercial collaborations to occur more efficiently, eliminating the traditional complexities associated with milestone negotiations and payment structures. Clients can directly access live mice for immunisation, significantly expediting the development of fully human therapeutic antibodies.
Features of NeoMab’s Technology
The NeoMab mice leverage human V(D)J gene-encoded antibodies, demonstrating a gene usage frequency and sequence diversity that closely mirrors that of humans. This genetic composition makes them ideal candidates for developing fully human antibodies. The mice possess a robust immune system capable of effective antigen presentation, antibody production, and affinity maturation. Additionally, the BALB/c genetic background and antigen presentation capabilities enhance the mice’s proficiency in presenting antigens, yielding serum titers reaching levels of 10^5 to 10^6 after just three to four rounds of immunisation.
Recent Surface Plasmon Resonance (SPR) testing has shown that the antibody affinities of NeoMab mice range from 10^-8 to 10^-10, catering to diverse requirements in fully humanised antibody development, thus establishing a solid foundation for pharmaceutical breakthroughs.
A New Collaboration Model
NeoMab’s commitment to providing convenient, one-stop services is evident in its innovative collaboration model. The NeoMab-IgG Ease platform effectively removes the need for lengthy negotiations and milestone payment processes that typically characterise traditional models. By allowing clients to obtain live mice directly for immunisation, the platform enables rapid development of fully human therapeutic antibodies and optimises the payment and agreement processes that are often cumbersome and time-consuming.
Advantages of Partnering with NeoMab
By leveraging the NeoMab platform, clients can access an array of fully human building blocks, including IgG, ScFv, and Fab, which can be tailored for various types of drug development and cell therapies. This includes Antibody-Drug Conjugates (ADCs), Antibody-Oligonucleotide Conjugates (AOCs), as well as mono-, bi-, and multispecific antibodies and nanobodies. NeoMab offers a comprehensive suite of services that spans from target validation to Investigational New Drug (IND) filing, ensuring a seamless experience throughout the experimental process.
Incorporating antibody screening with in vivo experimental animal models, NeoMab accelerates the in vivo study process and reduces the costs associated with humanisation. This capability enables clients to shorten their drug development timelines by as much as 1.5 years, allowing them to bring products to market faster. Many partners have recognised and praised NeoMab’s services for their role in expediting research and development processes, solidifying the company’s position as a leader in the field.
Conclusion
NeoMab’s pioneering NeoMab-IgG Ease model stands at the forefront of antibody drug development, offering unprecedented flexibility and efficiency. As the industry evolves, NeoMab remains dedicated to advancing the capabilities of fully human antibody discovery, ensuring that clients can navigate the complexities of drug development with confidence and optimism.